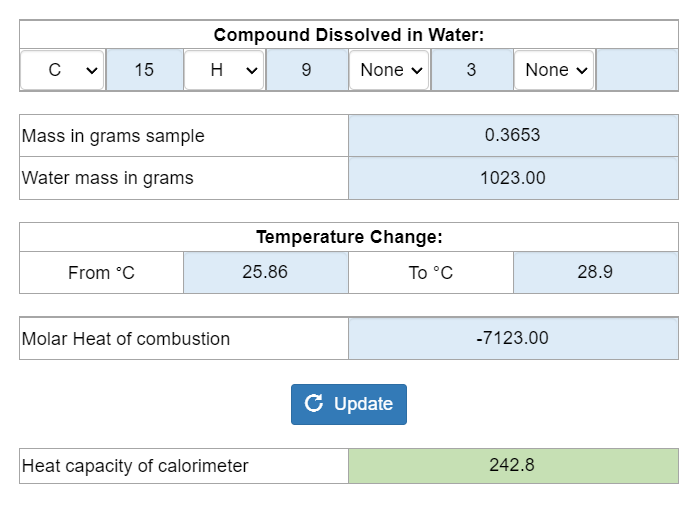
Bomb Calorimeter Specific Heat Capacity . then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of.
from coursestar.com
constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of.
Mastery Bomb Calorimetry Calculate Heat Capacity of Calorimeter
Bomb Calorimeter Specific Heat Capacity the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while.
From deboramilke.blogspot.com
Bomb Calorimetry Problems Debora Milke Bomb Calorimeter Specific Heat Capacity the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\). Bomb Calorimeter Specific Heat Capacity.
From socratic.org
When 0.602 g of biphenyl (C12H10) undergoes combustion in a bomb Bomb Calorimeter Specific Heat Capacity the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. . Bomb Calorimeter Specific Heat Capacity.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Bomb Calorimeter Specific Heat Capacity the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. heat capacity of the calorimeter system can be determined, allowing for the calculation of. Bomb Calorimeter Specific Heat Capacity.
From www.slideshare.net
2 pa32 bomb calorimeter procedure Bomb Calorimeter Specific Heat Capacity heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\).. Bomb Calorimeter Specific Heat Capacity.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Specific Heat Capacity heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume. Bomb Calorimeter Specific Heat Capacity.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Bomb Calorimeter Specific Heat Capacity the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\).. Bomb Calorimeter Specific Heat Capacity.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Specific Heat Capacity then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the. Bomb Calorimeter Specific Heat Capacity.
From www.slideserve.com
PPT Honors Physics PowerPoint Presentation, free download ID3195016 Bomb Calorimeter Specific Heat Capacity then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction. Bomb Calorimeter Specific Heat Capacity.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Bomb Calorimeter Specific Heat Capacity constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is. Bomb Calorimeter Specific Heat Capacity.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimeter Specific Heat Capacity the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume. Bomb Calorimeter Specific Heat Capacity.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Bomb Calorimeter Specific Heat Capacity heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the. Bomb Calorimeter Specific Heat Capacity.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Specific Heat Capacity the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. the temperature increase is measured and, along with the known heat capacity of the calorimeter,. Bomb Calorimeter Specific Heat Capacity.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3721051 Bomb Calorimeter Specific Heat Capacity heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine the heat of.. Bomb Calorimeter Specific Heat Capacity.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation ID3206969 Bomb Calorimeter Specific Heat Capacity the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. the bomb is placed in a water container and by measuring its temperature change. Bomb Calorimeter Specific Heat Capacity.
From www.youtube.com
Bomb Calorimetry Introduction Physical Chemistry Laboratory YouTube Bomb Calorimeter Specific Heat Capacity heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. then use equation \ref{12.3.1} to determine the heat capacity of the calorimeter (c bomb) from q comb and δt. then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the bomb is. Bomb Calorimeter Specific Heat Capacity.
From www.youtube.com
Bomb Calorimetry YouTube Bomb Calorimeter Specific Heat Capacity the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the. Bomb Calorimeter Specific Heat Capacity.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Bomb Calorimeter Specific Heat Capacity the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the bomb calorimeter is an instrument used to measure the heat of reaction at a fixed volume and the measured heat which is. heat capacity of the calorimeter system can be determined, allowing for the calculation of. Bomb Calorimeter Specific Heat Capacity.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry Bomb Calorimeter Specific Heat Capacity then use equation \(ref{5.5.9}\) to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). the temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. the bomb is placed in a water container and by measuring its temperature change caused by the reaction, we determine. Bomb Calorimeter Specific Heat Capacity.